From electronic health records to telemedicine, the digitization of healthcare in the countries of the European Union is progressing at different speeds and resembles a patchwork quilt. It is certainly true that there are a large number of promising European initiatives in the e-health sector. But a clear, pan-European vision is still lacking. In an impulse paper, we argue for an integrated e-health strategy. This blog post shows why and how such a strategy should be geared toward the needs of citizens.
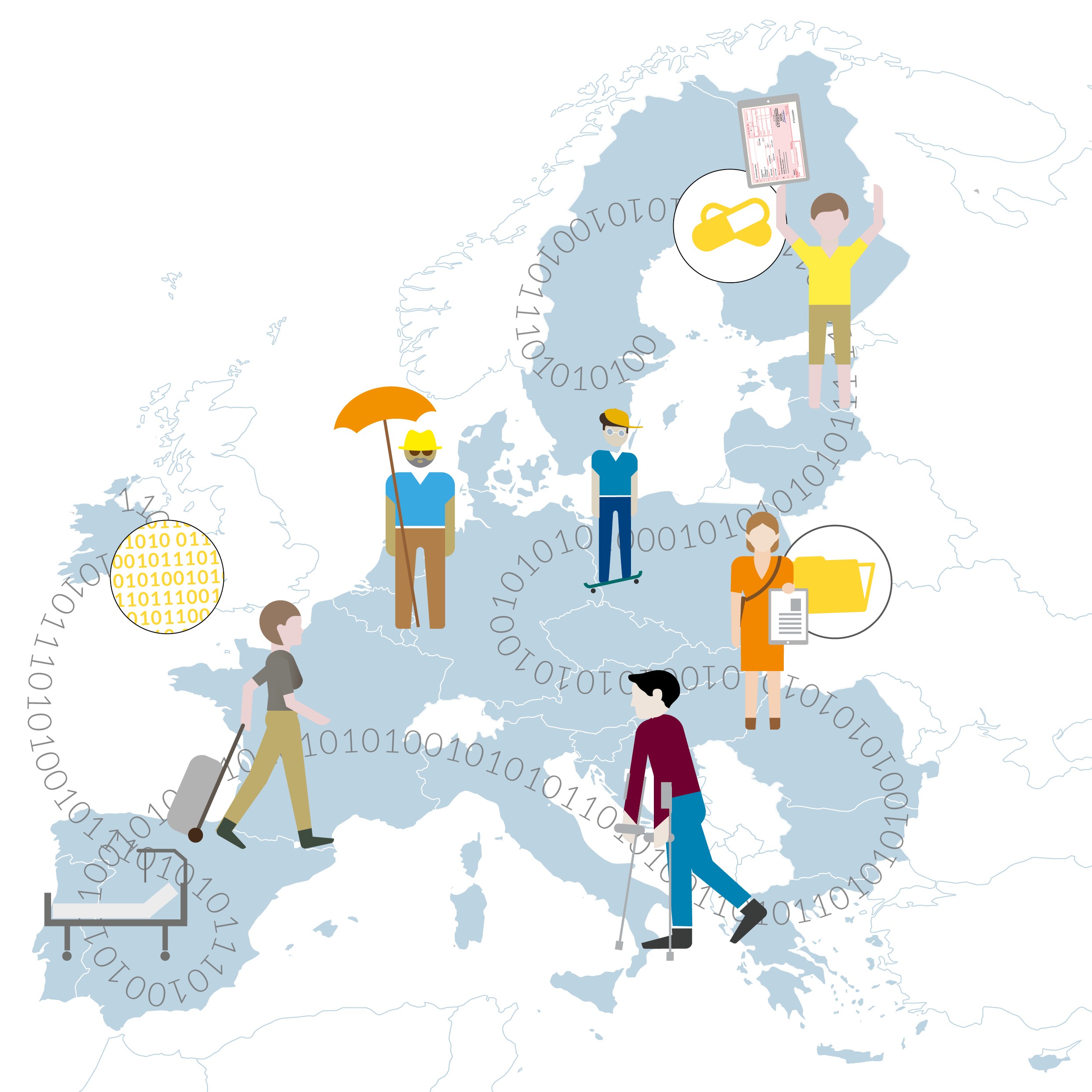
Skiing vacation in France. The slope was slippery. Suddenly slipped away. Broken arm. Hospitalization. Treatment on site. No problem: Back in Germany, the statutory health insurance reimburses the costs incurred. In principle, EU citizens have the right to receive healthcare services in any EU member state, the costs of which are reimbursed in their home country. An example of Europe in action.
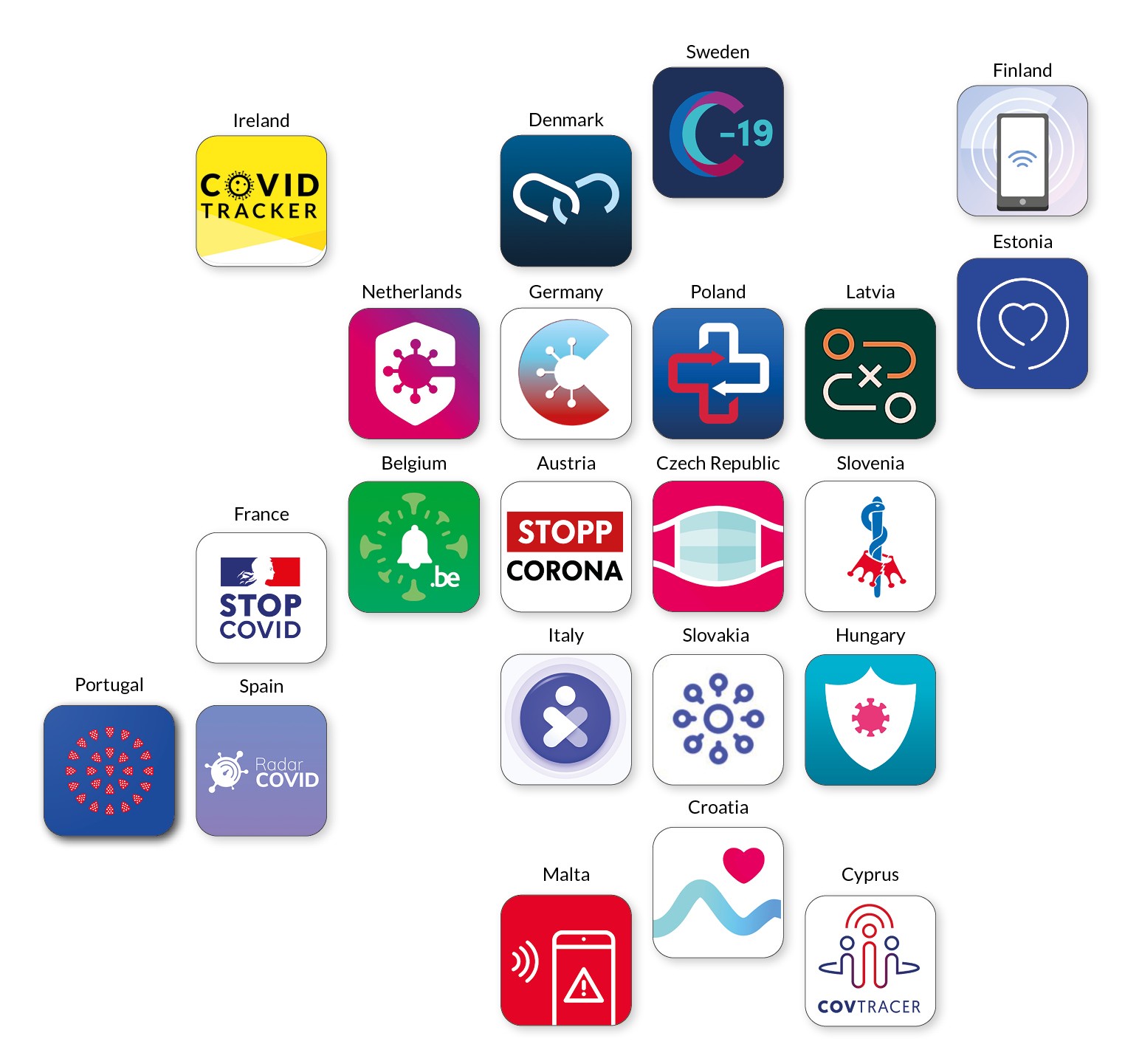
However, the situation is different in the e-health sector. For example, Corona tracking apps: To track infection chains, numerous EU countries have developed Corona apps. More than 20 such applications have been developed across the EU. However, for a long time, their rollout was largely carried out independently of each other. And thus a user of the German Corona warning app could initially only be warned of “German” risk contacts. Only since autumn has it been possible to exchange data between Corona apps from different EU member states. Yet a pandemic is a prime example of how integrated, transnational action offers far greater potential for sustainable and effective solutions than individual actions. It seems likely that a joint European app development from the outset could have done much more to contain the incidence of infection to date.
Great potential
However, quick joint action, which would be particularly advantageous in a pandemic with the risk of exponential growth, requires a prerequisite: a joint strategy must already be in place. For e-health, however, there are many useful individual strategies, but there is no clear, pan-European vision.
Yet an integrated strategy would have great potential to generate positive effects in various areas. It would be a cornerstone for a healthcare system that fully exploits the opportunities offered by digitization to make it easier for patients to access high-quality care.
In an impulse paper, we have compiled how an integrated European e-health strategy should be oriented toward the needs of citizens. First priority: It should be aligned in such a way that the digitization of European healthcare systems leads to noticeably better medical care for the general public in a timely manner.
E-health “Made in Europe” must be experienced
But how can the use of e-health be made an actual experience for Europe’s citizens? Take e-prescriptions, for example. For people living in border regions in particular, a cross-border solution for e-prescriptions would be a major advantage: they could easily redeem the e-prescription issued by their GP in their home country on their way to work in the neighboring member state. In Estonia and Finland, for instance, this is already possible: Finnish citizens can also use the e-prescriptions issued by their Finnish doctors in Estonian pharmacies, and pharmacies in Finland are able to redeem e-prescriptions from other EU countries.
A similar scenario would be the ski accident at the vacation destination: with a cross-border patient summary record, emergency services at the scene of the accident could easily access the accident victim’s electronic emergency data and know immediately whether he or she has allergies to painkillers, for example. The use of telemedicine from or within other EU countries could also function more smoothly thanks to such integrated strategic concepts.
However, these new approaches must meet the regulatory requirements of two or more healthcare systems. The challenge, therefore, is to find creative solutions and promote these consistently with a view to incorporating them into standard care. And: The regulations for the cross-border use of healthcare services should be adapted to the progress of digitization and reviewed to determine which digital healthcare services are to be covered.
“Made in Europe” as a trusted seal of quality and safety
The prerequisite for this, however, is that EU citizens can also trust that e-health “Made in Europe” is uniformly certified and safe for them – and functions in the same way everywhere. The EU Medical Devices Regulation and the European General Data Protection Regulation already provide a solid basis for achieving this. But there is still a lot of room for improvement in the cross-border exchange of health data, as well as in the evaluation and quality criteria used and the reimbursement rules for e-health applications. In other words: there is still a very high degree of heterogeneity across Europe. And approaches such as the German Digital Care Act (DVG), which allows doctors in Germany to prescribe digital therapies, hardly exist anywhere else in the EU.
It is not just the regulatory route that needs to be paved for an integrated EU strategy, however, in order to make it a tangible reality for EU citizens: A coordinated strategic effort is also needed when it comes to communication. EU citizens need to know: What are the health policy objectives of any such strategy? What are the regulatory principles? What about quality requirements and data protection in dealing with e-health?
The storyline is what truly matters
Clear communication formats are needed that are oriented toward the questions and needs of the individual target groups. Ultimately, the citizens are the users of digital technologies. This means they have to understand and be convinced of their added value. To do this, it must be clearly explained to citizens what benefits, and at the same time what risks, digital health solutions entail.
To this end, it is important, for example, to provide citizens with reliable, easily accessible information about the quality of health apps that are offered simultaneously in several member states. It is not enough, however, to simply explain that a product with a “Made in Europe” seal, for example, guarantees the protection of our data under a strict GDPR and that it is a guarantor of quality and security. Rather, people must also be emotionally engaged in order to ultimately become enthusiastic about the advantages of e-health – as well as the advantages of Europe. This can be done with the help of cross-border campaigns or extensive communication at local contact points on the part of service providers and insurers, who generally enjoy a high level of trust: The e-health Made in Europe storyline must be strong, address citizens directly – and highlight concretely how it will improve people’s health.
The Bertelsmann Stiftung’s impulse paper* contains a total of seven recommendations for action for an integrated European e-health strategy. We will discuss a number of them in more detail in a series of blog posts:
- An integrated European e-health strategy must be tailored to the needs of citizens.
- An integrated European e-health strategy must create a single e-health market.
- An integrated European e-health strategy must include the governance for a European health data space.
*Paper available in German with English recommendations for action.